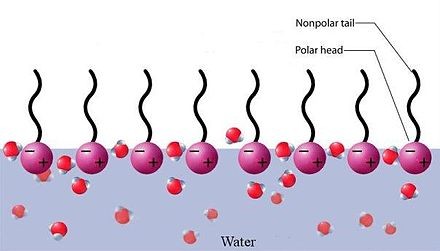
Surfactant is a wetting agent that reduces the surface tension between two liquids or between a liquid and a solid. A substance whose molecule is polar: a hydrophilic head and a hydrophobic tail.
Characteristics of surfactants
Surfactants are used to reduce the surface tension of a liquid by reducing the surface tension at the contact surface of two liquids. If more than two liquids are insoluble, the surfactant increases the contact area between the two liquids.
When a surfactant is mixed into a liquid, the surfactant molecules tend to form clusters (micelle, translated as micelles), the concentration at which the molecules begin to cluster is called is the critical clustering concentration. If the liquid is water , the molecules will clump together their hydrophobic tails and turn their hydrophilic heads to create different shapes such as spheres (0-dimensional), cylinders (1-dimensional), membranes (2-dimensional). .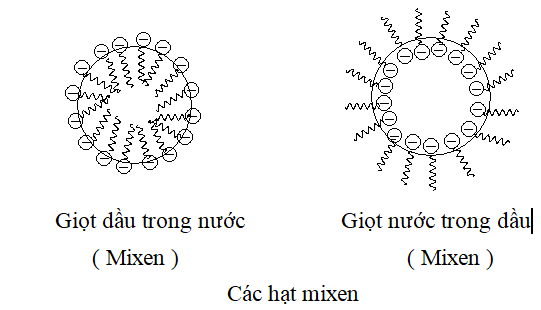
The hydrophilic, hydrophobicity of a surfactant is characterized by a parameter called the hydrophilic Lipophilic Balance (HLB), which can range from 0 to 40. The higher the HLB, the higher the HLB. the more dissolvable a chemical is in water, the lower the HLB, the easier it is to dissolve in non-polar solvents such as oil.
Classification of surfactants
Depending on the properties, surfactants are classified into different types. If looking at the electrical properties of the polar head of the surfactant molecule, they can be classified into the following categories:
-
Ion activator: when polarized, the polarizer is ionized
- Positive activator: when polarized, the polarizer is positively charged.
– Cetyl trimethylammonium bromide (CTAB)
– Cetyl pyridinium chloride (CPC)
– Polyethoxylated tallow amine (POEA)
– Benzalkonium chloride (BAC)
– Benzethonium chloride (BZT) - Negative activator: when polarized, the polarizer is negative charged.
– Sodium dodecyl sulfate (SDS), ammonium lauryl sulfate, and other alkyl sulfate salts
– Sodium laureth sulfate, or sodium lauryl ether sulfate (SLES)
– Alkyl benzene sulfonate
– Soaps and salts of fatty acids
- Positive activator: when polarized, the polarizer is positively charged.
-
Non-ionic activator: non-ionized polar head, e.g. Alkyl poly (ethylene oxide).
- Alkyl poly (ethylene oxide)
- Copolymers of poly (ethylene oxide) and poly (propylene oxide) (commercially known as Poloxamers or Poloxamines)
- Alkyl polyglucose, including:
– Octyl glucoside
– Decyl maltosite - Fatty Wines
– Cetyl alcohol
– Cleyl alcohol - Cocamit MEA, cocamite DEA
-
Bipolar activator: when polarized, the polarizer can be negatively or positively charged depending on the pH of the solvent.
-
- Dodecyl betaine
- Dodecyl dimethylamine oxide
- Cocamidopropyl betaine
- Coco amphor glycinate
-
Application
– Surfactants are widely used in daily life. The most common application is washing powder, painting, dying…


– In addition, applications in other fields such as:
In the textile dyeing industry: Fabric softener, dyeing auxiliaries.
In the food industry: Emulsifier for confectionery, dairy and canned goods.
In the cosmetic industry: Detergent, emulsifier, foaming agent.
In the printing industry: Auxiliary to absorb and disperse printing ink.
In agriculture: Substance for processing plant protection drugs.
In construction: Used to emulsify asphalt, increase the curing strength of concrete.
In petroleum: Drilling fluid emulsifier.
In mineral industry: As medicine flotation agent, emulsifier, foaming agent for mineral enrichment.
=>>> See also: What is a surfactant? Classification of surfactants<<<=